The Interplay Between Stress and Adrenal Health in Hypothyroidism
August 21, 2024
We ensure our content is always unique, unbiased, supported by evidence. Each piece is based on a review of research available at the time of publication and is fact-checked and medically reviewed by a topic expert.
Written by: Nada Ahmed
Medically reviewed by: Lara Zakaria PharmD, CNS, IFMCP
While systems like the hypothalamic-pituitary-adrenal (HPA) axis and the hypothalamic-pituitary-thyroid (HPT) axis regulate adrenal and thyroid hormones respectively, these systems are also interconnected and influence one another. (Kyriacou 2023)
Both acute and chronic stress affect thyroid function. (Puttaswamy 2024) Excess cortisol has been shown to suppress the central thyroid axis and result in thyroid dysfunction. (Cai 2020)(Wondisford 2015) This suppression of thyroid hormones may help the body conserve energy during times of high stress. (Engel 1972) At the same time, elevated cortisol concentrations in patients with severe hypothyroidism can also indicate a potential compensatory mechanism initiated by the HPA axis. (Sinha 2023)
There’s evidence that chronic cortisol deficiency may impair thyroid function. (Tamura 1995) Specifically, hypothyroidism accompanied by isolated adrenocorticotropic hormone (ACTH) deficiency appeared to be reversible after the replacement of the maintenance dose of glucocorticoid which led to an improvement of thyroid function. (Tamura 1995) This highlights that while excessive cortisol suppresses thyroid function, insufficient cortisol can also pose a problem since adequate levels are necessary for proper thyroid function. Glucocorticoids are necessary for normal bodily functions which is why glucocorticoid receptors are present in almost all tissues in the body and are able to affect nearly every organ system. (Thau 2023)(Chourpiliadis 2023) This points to the importance of balanced adrenal health and cortisol production for normal thyroid function.
Research has shown that middle-aged people today are generally more stressed than they were in the past. (Almeida 2020) With high stress being the norm, evidence shows that high cortisol can affect thyroid hormone production and the risk of thyroid conditions.
Cortisol levels impact the production and conversion of thyroid hormones as well as the sensitivity of tissues to thyroid hormones.
Cortisol can affect the HPT axis, which regulates thyroid hormone production. High cortisol levels may suppress the release of thyroid-stimulating hormone (TSH) from the pituitary gland, thereby reducing thyroid hormone synthesis and secretion. (Cai 2020)(Wondisford 2015)
In addition, studies have shown that perceived stress and psychosocial stress are able to elicit a significant increase in TSH. (Fischer 2019)(Puttaswamy 2024) The positive correlation observed between TSH and cortisol is a result of the negative feedback to lower levels of thyroid hormone brought on by stress. (Sinha 2023)
Apart from suppression of the thyroid as an adaptive response to stress, another possible mechanism for decreased thyroid hormone may be due to reduced availability of tyrosine for thyroid hormone synthesis. (Engel 1972)(Polak 2013) Tyrosine is a precursor for the synthesis of catecholamines—epinephrine and norepinephrine—which are also released as part of the stress response. (Thau 2023)(Young 2007) With increased stress, these catecholamines can be depleted and tyrosine availability could theoretically affect thyroid function. (van Spronsen 2001)(Young 2007) Research demonstrates that supplementation of tyrosine improves catecholamine levels and thyroid hormone markers. (Palinkas 2007)(Young 2007)
Higher levels of serum cortisol are associated with decreases in peripheral triiodothyronine (T3) and thyroxine (T4), as cortisol can inhibit the peripheral conversion of T4 to T3 and increase reverse T3 (rT3), a less bioactive form of thyroid hormone. (Helmreich 2011)(Holtorf 2014)(McCormack 1998)(Sinha 2023) Elevated cortisol levels may impair the activity of enzymes involved in this conversion, potentially reducing T3 levels despite normal T4 production. (Paragliola 2021)
Given that cortisol can impact blood sugar regulation, it can also affect the sensitivity of tissues to thyroid hormones. As part of the stress response, blood sugar levels increase. While this is adaptive, with chronic stress, it can lead to insulin resistance and diabetes. (Sharma 2022) Evidence demonstrates that resistance to thyroid hormone indices is associated with obesity, metabolic syndrome, and diabetes. (Laclaustra 2018) Thus, high cortisol levels can decrease the sensitivity of tissues to thyroid hormones and reduce metabolic activity as a result.
 Among the negative effects of chronic stress on our health, thyroid hormone production and regulation can also be impacted.
Among the negative effects of chronic stress on our health, thyroid hormone production and regulation can also be impacted.
Psychological and physiological stressors induce immune modulations that can lead to autoimmune thyroid diseases, resulting in hypothyroidism. (Puttaswamy 2024) Numerous studies have established the negative impact of psychological and physiologic stressors on immune function through various mechanisms. (Mizokami 2004)(Puttaswamy 2024) These immune modulations may contribute to the development of autoimmunity, increase the susceptibility to autoimmune disease in genetically predisposed individuals, and lead to autoimmune thyroid diseases. (Mizokami 2004)(Puttaswamy 2024)
Previous research has indicated that critical life events often precede the onset of autoimmune thyroid diseases. (Fischer 2019) In this way, stress can be an environmental trigger for the onset of Hashimoto's thyroiditis or Grave’s disease. (Wang 2023)
It’s important to assess adrenal health in the context of thyroid dysfunction, given the role that adrenal imbalances can play in thyroid function. Additionally, symptoms of adrenal insufficiency can look like symptoms of hypothyroidism, so consider adrenal function when identifying the root cause of the pathology. (JCDR 2024) Several clinical studies have shown that balancing cortisol levels can aid in the recovery of normal hypothalamus-pituitary-thyroid axis activity. (Paragliola 2021)
Common signs and symptoms of excess cortisol include:
Central weight gain
Diabetes
Diffuse cutaneous hyperpigmentation
Easy bruising and cutaneous atrophy
Enlarged dorsocervical pad (buffalo hump) and supraclavicular fat pads
Facial rounding or moon facies
Hair loss
Headaches, dizziness, and visual blurring due to blood pressure elevations
Hirsutism in women
Hypertension
Marked acne and facial flushing including marked malar telangiectasia
Menstrual irregularities, including oligomenorrhea and amenorrhea
Osteoporosis
Polyuria, polydipsia, and either polyphagia or anorexia due to worsening hyperglycemia
Poor mood
Proximal muscle weakness
Sleep disturbances (Thau 2023)(Uwaifo 2023)
Note that it’s important to rule out Cushing’s disease or hypercortisolism, which involves high levels of cortisol due to causes such as ACTH-secreting pituitary adenoma or excessive oral or injectable corticosteroid use. (Thau 2023)
Symptoms of low cortisol are more non-specific and include:
Abdominal pain
Chronic, or long-lasting, fatigue
Cravings for salty foods
Diarrhea
Hyperpigmentation of the skin
Hypoglycemia, or low blood glucose
Irregular or no menstrual periods
Irritability and depression
Joint pain
Loss of appetite
Loss of interest in sex
Low blood pressure that drops further when you stand up, causing dizziness or fainting
Muscle weakness
Nausea
Vomiting
Weight loss (NIDDK 2022)(Thau 2023)
Note that it’s important to rule out causes of low cortisol such as Addison’s disease or chronic adrenal insufficiency, most commonly caused by autoimmune destruction of the adrenal cortex. (Thau 2023)
Laboratory testing is essential for accurately diagnosing and managing thyroid disorders. These tests can uncover specific markers and hormone levels that provide a detailed picture alongside thyroid function, helping to identify contributing factors to conditions such as hypothyroidism, hyperthyroidism, and autoimmune thyroid diseases like Hashimoto's and Graves' disease.
Conducting testing for adrenal function can help tailor an approach that ensures personalized and effective care, leading to improved patient outcomes.
Cortisol testing is a procedure used to measure the levels of cortisol in a person's blood, urine, or saliva. Testing can help gain insight into how a person’s cortisol levels could be affecting their thyroid, as cortisol is one of the major hormones involved in stress response. (Thau 2023)
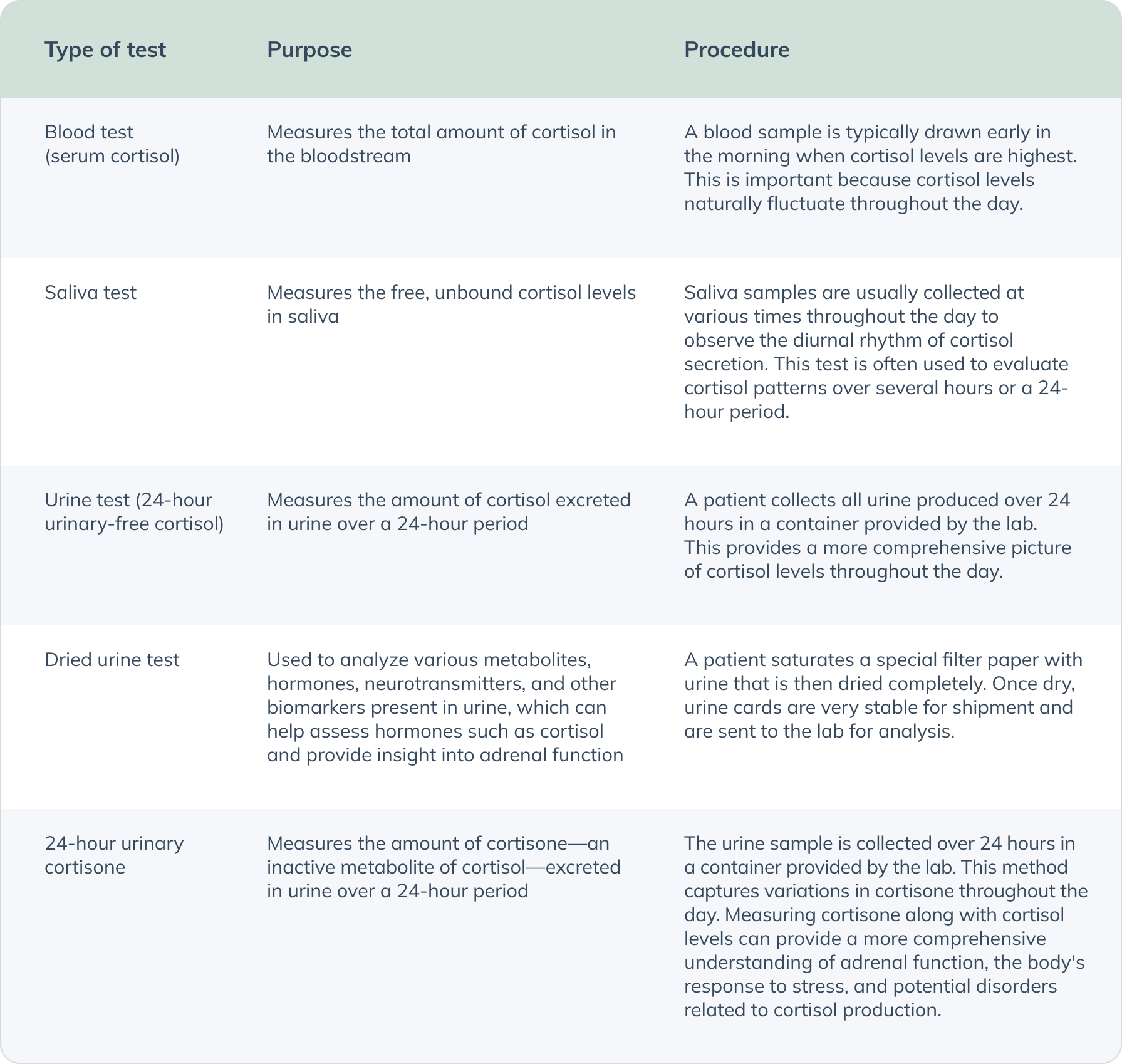
Cortisol levels vary throughout the day, following a natural circadian rhythm—typically peaking right before waking, decreasing towards the evening, and reaching the lowest levels around midnight.
Normal ranges can vary between labs, but deviations from the expected diurnal rhythm of cortisol (i.e., low cortisol in the morning or elevated cortisol at night) or extremely high or low levels may indicate underlying health issues. While cortisol pattern is regulated by the HPA axis, chronic stress can disrupt the normal diurnal rhythm of cortisol, leading to elevated or flattened cortisol levels throughout the day. High levels of cortisol associated with increased stress has the potential to then suppress the HPT axis and decrease peripheral conversion and cellular sensitivity to thyroid hormone. High cortisol may also indicate conditions like Cushing's syndrome or result from use of certain medications. (Azmi 2021)(Chan 2010)
To help rule out more serious causes for adrenal dysfunction, other tests such as serum ACTH, the dexamethasone suppression test, and the ACTH stimulation test can be used to provide further insight into the HPA axis. Low cortisol levels may suggest adrenal insufficiency and prolonged stress response but may also be indicative of Addison's disease.
Organic acid tests (OATs) are valuable tools in assessing adrenal function within the context of a comprehensive thyroid assessment. These tests measure the metabolites in urine that are byproducts of various metabolic processes, including those involved in the stress response and adrenal function. OATs can offer a broader perspective on adrenal function compared to solely testing cortisol hormone levels, as they look at metabolites of various hormones that can be involved in the stress response. Here are some key organic acids related to adrenal function that OATs evaluate:
Vanilmandelate (VMA): VMA is a primary metabolite of catecholamines (epinephrine and norepinephrine). Elevated levels can indicate increased adrenal activity and chronic stress. (Mount Sinai n.d)
Homovanillate (HVA): HVA is a major metabolite of dopamine. Abnormal levels can indicate dysregulation in dopamine metabolism, often linked to stress and adrenal function. (Mount Sinai n.d)
5-Hydroxyindoleacetic acid (5-HIAA): 5-HIAA is a metabolite of serotonin, a neurotransmitter that influences mood and stress response. Abnormal levels of 5-HIAA can indicate issues with serotonin metabolism, often related to stress and adrenal function. (Khan 1988)
Kynurenic acid: This metabolite is part of the tryptophan metabolism pathway. High levels can indicate chronic stress and inflammation, as the pathway shifts from producing serotonin to kynurenine during stress. Additionally, elevated kynurenine levels may indicate a deficiency in vitamin B6, as B6 is a cofactor in converting kynurenine to kynurenic acid. This imbalance can further impact the stress response and adrenal function. (La Torre 2021)
Quinolinate: Quinolinate is another metabolite in the tryptophan pathway. Elevated levels of quinolinate are associated with neuroinflammation and can reflect chronic stress and adrenal dysfunction. (Saade 2022)
By including OATs in a comprehensive thyroid assessment, healthcare providers can gain insights into the patient's stress response and adrenal function. Elevated levels of these metabolites can indicate chronic stress, which can disrupt the HPT axis and affect thyroid hormone balance. This information is crucial for developing a holistic and effective treatment plan that addresses both thyroid and adrenal health, ensuring better overall endocrine function and improved patient outcomes.
The relationship between stress and adrenal health in hypothyroidism highlights a complex interplay. Chronic stress can contribute to thyroid dysfunction and exacerbate hypothyroid symptoms. Adrenal glands, crucial for regulating stress response and cortisol production, may become dysregulated due to chronic stress, potentially leading to burnout or dysfunction. This dysregulation can further impair thyroid hormone function, exacerbating hypothyroid symptoms.
Managing stress effectively is therefore essential in supporting adrenal health and optimizing thyroid function in individuals with hypothyroidism and autoimmune thyroid disease.


References
Almeida, D. M., Charles, S. T., Mogle, J., Drewelies, J., Aldwin, C. M., Spiro, A., & Gerstorf, D. (2020). Charting adult development through (historically changing) daily stress processes. The American psychologist, 75(4), 511–524. https://doi.org/10.1037/amp0000597
Azmi, N. a. S. M., Juliana, N., Azmani, S., Effendy, N. M., Abu, I. F., Teng, N. I. M. F., & Das, S. (2021). Cortisol on circadian rhythm and its effect on cardiovascular system. International Journal of Environmental Research and Public Health, 18(2), 676. https://doi.org/10.3390/ijerph18020676
Cai, R., Zhou, W., Jiang, L., Jiang, Y., Su, T., Zhang, C., Zhou, W., Ning, G., & Wang, W. (2020). Association between thyroid function and serum cortisol in cortisol-producing adenoma patients. Endocrine, 69(1), 196–203. https://doi.org/10.1007/s12020-020-02278-5
Chan, S., & Debono, M. (2010). Replication of cortisol circadian rhythm: new advances in hydrocortisone replacement therapy. Therapeutic advances in endocrinology and metabolism, 1(3), 129–138. https://doi.org/10.1177/2042018810380214
Chourpiliadis, C., & Aeddula, N. R. (2023, July 17). Physiology, glucocorticoids. StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK560897/
Dexamethasone suppression test. (n.d.). Mount Sinai Health System. https://www.mountsinai.org/health-library/tests/dexamethasone-suppression-test
Engel, G. L., & Schmale, A. H. (1972). Conservation-withdrawal: a primary regulatory process for organismic homeostasis. Ciba Foundation symposium, 8, 57–75. https://doi.org/10.1002/9780470719916.ch5
Fischer, S., Strahler, J., Markert, C., Skoluda, N., Doerr, J. M., Kappert, M., & Nater, U. M. (2019). Effects of acute psychosocial stress on the hypothalamic-pituitary-thyroid (HPT) axis in healthy women. Psychoneuroendocrinology, 110, 104438. https://doi.org/10.1016/j.psyneuen.2019.104438
Helmreich, D. L., & Tylee, D. (2011). Thyroid hormone regulation by stress and behavioral differences in adult male rats. Hormones and behavior, 60(3), 284–291. https://doi.org/10.1016/j.yhbeh.2011.06.003
Holtorf, K. (2014). Peripheral thyroid hormone conversion and its impact on TSH and metabolic activity. Journal of Restorative Medicine, 3(1), 30–52. https://doi.org/10.14200/jrm.2014.3.0103
JCDR - Adrenal insufficiency, hypothyroidism. (n.d.). https://www.jcdr.net/article_fulltext.asp?id=851
Khan, N. A., & Kalra, V. (1988). Urinary 5-HIAA and VMA and their relationship with immunoregulatory cells in stress administered subjects. Archivum immunologiae et therapiae experimentalis, 36(6), 717–721. https://pubmed.ncbi.nlm.nih.gov/2473718/
Khare, S., & Anjum, F. (2023, May 1). Adrenocorticotropic hormone (Cosyntropin) stimulation test. StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK555940/
Kyriacou, A., Tziaferi, V., & Toumba, M. (2023). Stress, Thyroid Dysregulation, and Thyroid Cancer in Children and Adolescents: Proposed Impending Mechanisms. Hormone research in paediatrics, 96(1), 44–53. https://doi.org/10.1159/000524477
La Torre, D., Dalile, B., De Loor, H., Van Oudenhove, L., & Verbeke, K. (2021). Changes in kynurenine pathway metabolites after acute psychosocial stress in healthy males: a single-arm pilot study. Stress, 24(6), 920–930. https://doi.org/10.1080/10253890.2021.1959546
Laclaustra, M., Moreno-Franco, B., Lou-Bonafonte, J. M., Mateo-Gallego, R., Casasnovas, J. A., Guallar-Castillon, P., Cenarro, A., & Civeira, F. (2018). Impaired sensitivity to thyroid hormones is associated with diabetes and metabolic syndrome. Diabetes Care, 42(2), 303–310. https://doi.org/10.2337/dc18-1410
McCormack, P. D., Thomas, J., Malik, M., & Staschen, C. M. (1998). Cold stress, reverse T3 and lymphocyte function. Alaska medicine, 40(3), 55–62. https://pubmed.ncbi.nlm.nih.gov/9785613/
Mizokami, T., Wu Li, A., El-Kaissi, S., & Wall, J. R. (2004). Stress and thyroid autoimmunity. Thyroid : official journal of the American Thyroid Association, 14(12), 1047–1055. https://doi.org/10.1089/thy.2004.14.1047
Mount Sinai. (n.d.). Catecholamines - urine. Mount Sinai Health System. https://www.mountsinai.org/health-library/tests/catecholamines-urine
NIDDK. (2022, November 8). Symptoms Causes of Adrenal Insufficiency & Addison’s Disease. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/endocrine-diseases/adrenal-insufficiency-addisons-disease/symptoms-causes
Palinkas, L. A., Reedy, K. R., Smith, M., Anghel, M., Steel, G. D., Reeves, D., Shurtleff, D., Case, H. S., Van Do, N., & Reed, H. L. (2007). Psychoneuroendocrine effects of combined thyroxine and triiodothyronine versus tyrosine during prolonged Antarctic residence. International journal of circumpolar health, 66(5), 401–417. https://pubmed.ncbi.nlm.nih.gov/18274206/
Paragliola, R. M., Corsello, A., Papi, G., Pontecorvi, A., & Corsello, S. M. (2021). Cushing's Syndrome Effects on the Thyroid. International journal of molecular sciences, 22(6), 3131. https://doi.org/10.3390/ijms22063131
Polak, M., & Szinnai, G. (2013). Thyroid disorders. In Elsevier eBooks (pp. 1–24). https://doi.org/10.1016/b978-0-12-383834-6.00088-4
Puttaswamy, S. H., Nandibewur, N. P., Kumar, P., Venkataiah, V., & Pinjar, M. J. (2024). A Cross-Sectional Study of the Relationship Between Perceived Stress and Thyroid Function Among Apparently Normal Women in the Reproductive Age. Cureus, 16(3), e55567. https://doi.org/10.7759/cureus.55567
Saade, M. C., Clark, A. J., & Parikh, S. M. (2022). States of quinolinic acid excess in urine: A systematic review of human studies. Frontiers in nutrition, 9, 1070435. https://doi.org/10.3389/fnut.2022.1070435
Sharma, K., Akre, S., Chakole, S., & Wanjari, M. B. (2022). Stress-Induced Diabetes: A Review. Cureus, 14(9), e29142. https://doi.org/10.7759/cureus.29142
Sinha, S. R., Prakash, P., Keshari, J. R., Kumari, R., & Prakash, V. (2023). Assessment of Serum Cortisol Levels in Hypothyroidism Patients: A Cross-Sectional Study. Cureus, 15(12), e50199. https://doi.org/10.7759/cureus.50199
Tamura, M., Yokoyama, N., Nishikawa, T., Takeshita, A., Kimura, H., Ashizawa, K., Kiriyama, T., & Nagataki, S. (1995). Improvement of hypothyroidism after glucocorticoid replacement in isolated adrenocorticotropin deficiency. Internal medicine (Tokyo, Japan), 34(6), 559–563. https://doi.org/10.2169/internalmedicine.34.559
Thau, L., Gandhi, J., & Sharma, S. (2023, August 28). Physiology, cortisol. StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK538239/
Tohei, A. (2004). Studies on the Functional Relationship between Thyroid, Adrenal and Gonadal Hormones. Journal of Reproduction and Development, 50(1), 9–20. https://doi.org/10.1262/jrd.50.9
Uwaifo, G. I., & Hura, D. E. (2023, July 4). Hypercortisolism. StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK551526/
Van Spronsen, F. J., van Rijn, M., Bekhof, J., Koch, R., & Smit, P. G. (2001). Phenylketonuria: tyrosine supplementation in phenylalanine-restricted diets. The American journal of clinical nutrition, 73(2), 153–157. https://doi.org/10.1093/ajcn/73.2.153
Wang, J., Chen, Z., Carru, C., Capobianco, G., Sedda, S., & Li, Z. (2023). What is the impact of stress on the onset and anti-thyroid drug therapy in patients with graves' disease: a systematic review and meta-analysis. BMC endocrine disorders, 23(1), 194. https://doi.org/10.1186/s12902-023-01450-y
Wondisford F. E. (2015). A direct role for thyroid hormone in development of the adrenal cortex. Endocrinology, 156(6), 1939–1940. https://doi.org/10.1210/en.2015-1351
Young S. N. (2007). L-tyrosine to alleviate the effects of stress?. Journal of psychiatry & neuroscience : JPN, 32(3), 224. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1863555/